Lithium-ion batteries are widely used in various applications, including electric vehicles, consumer electronics, and energy storage systems, due to their high energy density, long cycle life, and efficiency. A crucial component of these batteries is the electrolyte, which plays a key role in transporting lithium ions between the electrodes and ensuring stable battery performance. Understanding how lithium-ion batteries and their electrolytes work can help improve battery safety, longevity, and overall efficiency.
How Does a Lithium-Ion Battery Work?
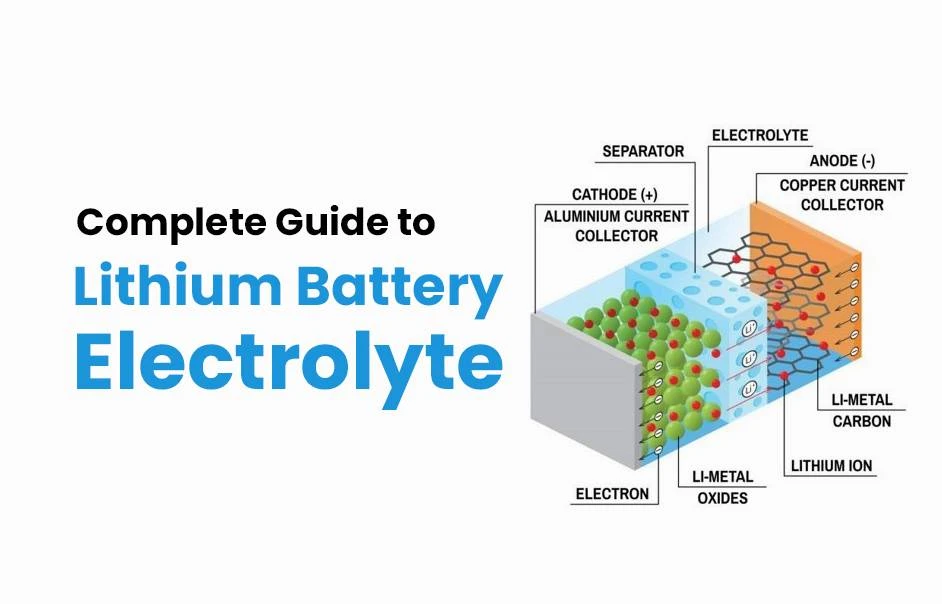
A lithium-ion battery stores and releases electrical energy through the migration of lithium ions (Li⁺) between the positive and negative electrodes:
- Charging Process: An external voltage forces lithium ions to detach from the cathode (e.g., LiCoO₂), migrate through the electrolyte to the anode (e.g., graphite), and embed into its structure, while electrons flow through the external circuit to the anode.
- Discharging Process: Lithium ions leave the anode and return to the cathode, while electrons travel through the external circuit to perform work (e.g., powering a device).
- Electrolyte Function: It provides an ion transport pathway and stabilizes the electrode/electrolyte interface.
What Is Battery Electrolyte?
The electrolyte is the ion-conducting medium in a battery, typically composed of a solvent, lithium salt, and additives. Its functions include:
- Transporting lithium ions between the cathode and anode.
- Forming a stable Solid Electrolyte Interphase (SEI) to protect electrode materials.
- Maintaining the stability of electrochemical reactions.
Basic Requirements for Lithium-Ion Battery Electrolyte
- High Ionic Conductivity (>1 mS/cm): Ensures fast charging and discharging.
- Wide Electrochemical Window (>4.5 V): Withstands high-voltage cathode materials.
- Chemical Stability: Avoids side reactions with electrode materials or current collectors.
- Thermal Stability: Does not decompose or catch fire at high temperatures.
- Low-Temperature Adaptability: Maintains fluidity at low temperatures.
- Environmental Friendliness: Low toxicity and biodegradable.
What Is Lithium-Ion Battery Electrolyte Made Of?
- Solvents (~80%):
- Carbonates: Such as ethylene carbonate (EC) and dimethyl carbonate (DMC), which dissolve lithium salts.
- Lithium Salts (~10-15%):
- Lithium hexafluorophosphate (LiPF₆): The mainstream lithium salt, though it has poor thermal stability.
- New lithium salts: Such as lithium bis(fluorosulfonyl)imide (LiFSI), which offer better thermal stability.
- Additives (~5%):
- Film-forming additives (e.g., VC): Optimize the SEI layer.
- Flame retardants (e.g., phosphates): Improve safety.
- Overcharge protectors (e.g., biphenyl): Prevent battery overcharging.
Types of Lithium-Ion Battery Electrolytes
| Type | Composition | Advantages | Disadvantages |
|---|---|---|---|
| Liquid Electrolyte | Carbonate solvent + LiPF₆ + additives | High ionic conductivity, low cost | Flammable, leakage risk, thermal decomposition |
| Solid Electrolyte | Oxides (LLZO), sulfides (LPS), etc. | High safety, compatible with lithium metal anodes | High interfacial resistance, high production cost |
| Gel Electrolyte | Liquid electrolyte + polymer (e.g., PVDF) | Balances safety and ionic conductivity | Lower mechanical strength |
How Does Electrolyte Affect Battery Performance?
- Capacity and Rate Performance: Electrolytes with low conductivity increase internal resistance, reducing discharge capacity.
- Cycle Life: An unstable SEI layer accelerates electrode degradation.
- Safety: Flammable electrolytes increase the risk of thermal runaway (e.g., fire, explosion).
- Temperature Adaptability: Electrolytes can freeze or thicken at low temperatures, causing battery failure.
- Energy Density: High-voltage electrolytes are needed for high-energy-density electrode materials (e.g., NCM811).
Ideal Standards for Lithium Battery Electrolyte
- Ultra-high ionic conductivity (>10 mS/cm).
- High-voltage resistance (>5 V) with strong oxidation/reduction stability.
- Wide operating temperature range (-40°C to 120°C).
- Non-flammable and non-toxic.
- Compatible with lithium metal anodes to suppress dendrite growth.
- Low-cost and easily recyclable.
Q&A (Frequently Asked Questions)
Q1: How is electrolyte different from regular liquid?
A1: Electrolyte is an ionic conductor that contains dissociable lithium salts, whereas regular liquids (e.g., water) do not conduct electricity or have very low conductivity.
Q2: Can electrolyte be replaced by other liquids?
A2: No. Regular liquids cannot provide a lithium-ion transport pathway and may cause side reactions (e.g., decomposition, gas formation).
Q3: What happens if the electrolyte leaks?
A3: Leakage can cause short circuits, damage equipment, or even trigger fires (since liquid electrolytes contain flammable solvents).
Q4: Why are solid-state electrolytes safer?
A4: Solid electrolytes are non-flammable and can physically block lithium dendrite penetration, preventing short circuits.
Q5: How will electrolytes evolve in the future?
A5: Future development will focus on solid-state electrolytes, high-voltage compatibility, and environmental friendliness, such as sulfur-based solid electrolytes or bio-based solvents.
By optimizing electrolyte design, the performance and safety of lithium-ion batteries will continue to improve, driving advancements in electric vehicles, energy storage systems, and beyond.
This translation maintains technical accuracy while ensuring readability. Let me know if you need any refinements! 🚀


